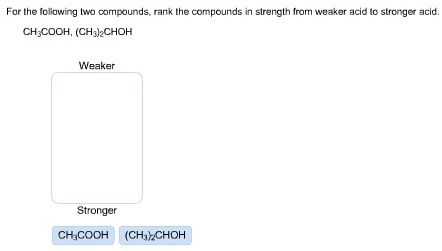
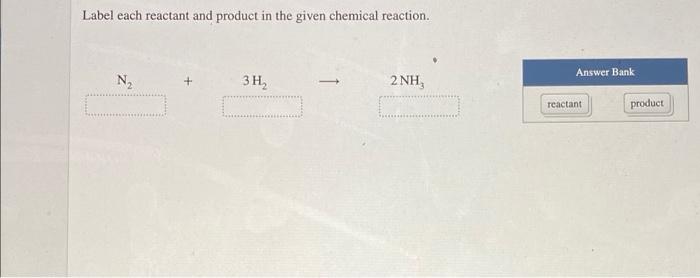
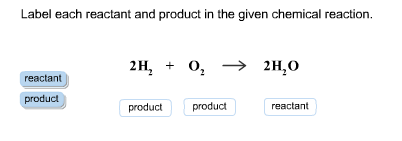
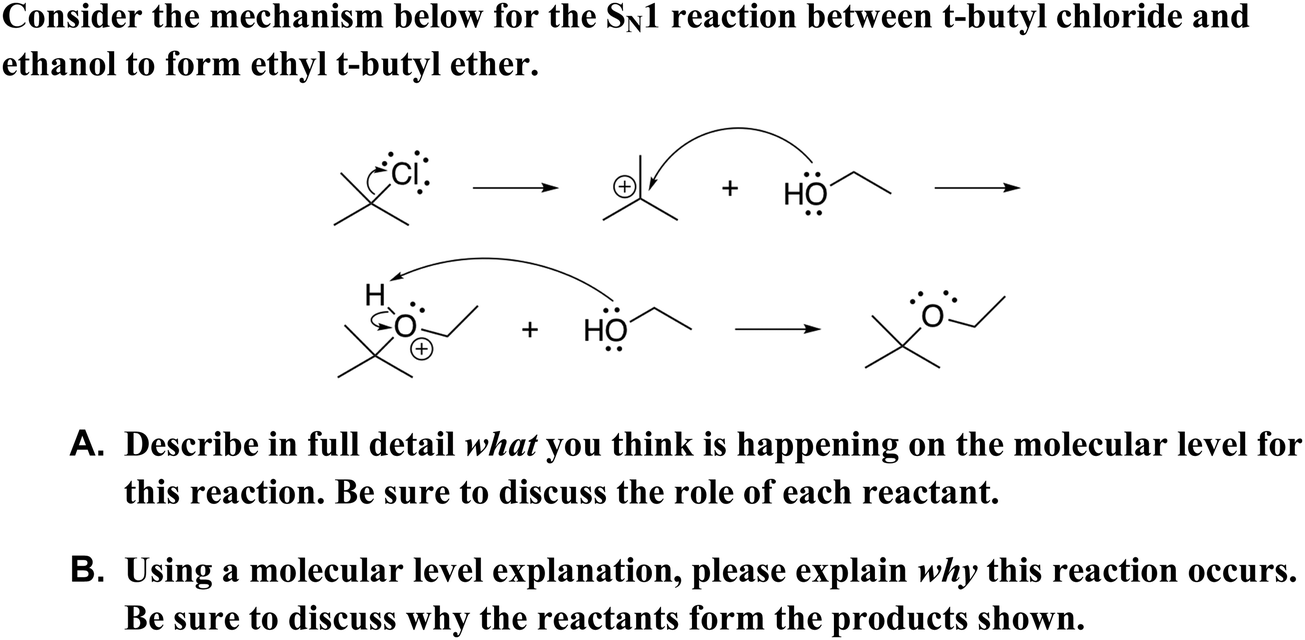
43 label each reactant and product in the given chemical reaction
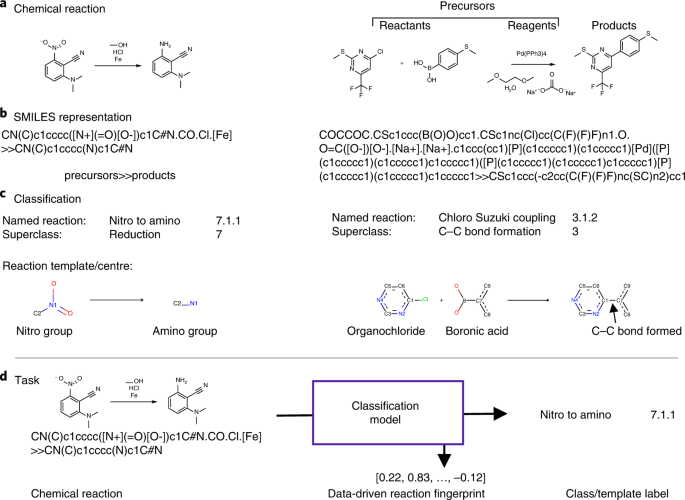
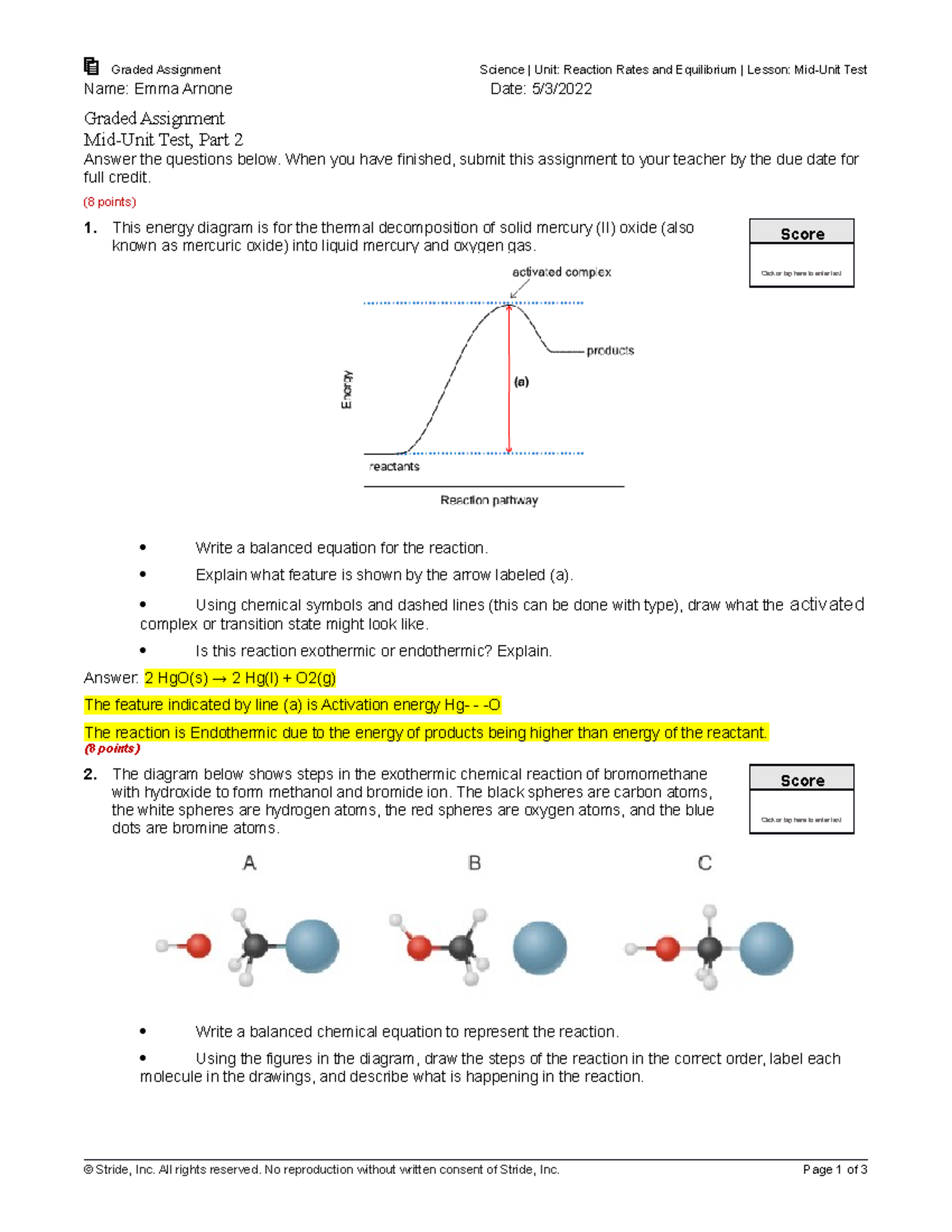
Daylight Theory: SMILES Component parts of a reaction are handled by introducing the ">" character as a new separator. Any reaction must have exactly two > characters in it. ">>" is a valid reaction SMILES for an empty reaction. Each of the ">"-separated components of a reaction must be a valid molecule SMILES. As an aside, molecule SMILES never have a ">" character ... 2.2.5.b (2).docx - a. Label the reactants and the products... e. In the space below, draw a potential energy diagram for an endothermic reaction and a second potential energy diagram for an exothermic reaction. Give each graph a title, and label the reactants and products on each graph. (2 points) Question 2 (7 points) a. What field of physics involves studying heat, work, and energy?
Organic Chemistry Q&A.docx - 1. Label each reactant... View Organic Chemistry Q&A.docx from CHEM 1032 at University of Belize - Belmopan. 1. Label each reactant according to its role (or roles) in the chemical reaction. Check all that apply. 2. Add

Label each reactant and product in the given chemical reaction
Answered: Label each reactant and product in this… | bartleby Q: Identify each reactant and product in this reaction as a Brønsted acid or base. H2Y− + H2Z−↽−−⇀H3Y +…. A: Q: From the following list, please choose the conjugate acid of NH3' - CH2 - O NH3* - CHR - COOH O NH3*…. A: Acids are substances that release H+ ions. Bases are substances that accepts H+ ions. Note: Since…. Solved Label each reactant and product in the given chemical | Chegg.com Expert Answer. CH4=REACTANT O2 = REACT …. View the full answer. Transcribed image text: Label each reactant and product in the given chemical reaction. CH_4 + 2O_2 rightarrow CO_2 + 2H_2O. 14.3: Effect of Concentration on Reaction Rates: The Rate Law 03/02/2022 · For each reaction, give the units of the rate constant, give the reaction order with respect to each reactant, give the overall reaction order, and predict what happens to the reaction rate when the concentration of the first species in each chemical equation is doubled. \(\mathrm{2HI(g)}\xrightarrow{\textrm{Pt}}\mathrm{H_2(g)}+\mathrm{I_2(g)}
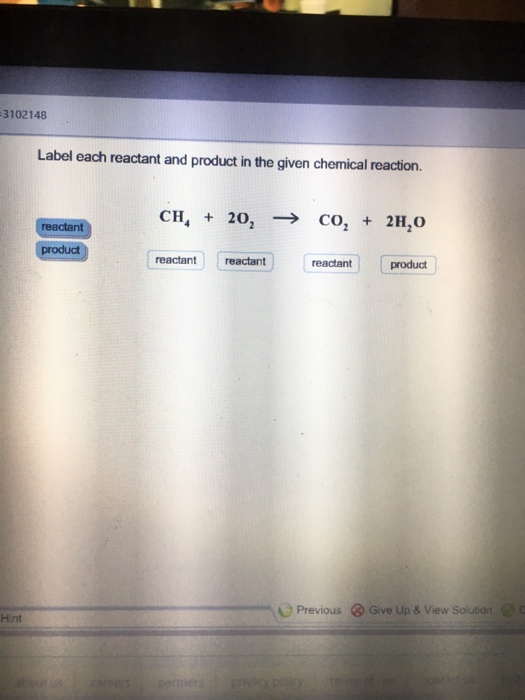
Label each reactant and product in the given chemical reaction. Solved 1. Label each reactant and product in the given | Chegg.com 1. Label each reactant and product in the given chemical reaction. CH4 + 2O2 CO2+ 2H2OCH4 + 2O2 CO2 + 2H2O Answer Bank: Product/Reactant 2. Use the law of conservation of mass to answer the questions. Consider a hypothetical reaction in which A and B are reactants and C and D are products. Background Kinetics is the study of rates and | Chegg.com The reactants may in fact react to form a number of intermediate species which in turn react with each other or more reactant to form the product. The existence of such intermediate species contributes to the overall rate of reaction and may even be ratecontrolling. In many reactions the rate-limiting step determines the overall rate and the addition of more reactant has little or no … [Solved] How does knowing the reactants and products help you classify ... For instance, when you are given two reactants and only one product is made then you can classify it as a synthesis reaction. Another would be if an organic compound is readteed with oxygen, CO2 and water is produced then you can classify it as a combustion reaction. Advertisement. Advertisement. Foundations of College Chemistry, Alternate Morris Hein, Susan Arena · 2010 · Science(b) Write a balanced formula equation including the state for each substance. (c) Label each reactant and product to show the relative amounts of each ...
Answered: Classify each reactant and product in… | bartleby Classify each reactant and product in the reaction as an acid or base according to the Brønsted theory. + H2ö = ÖH + Acid Base C,H,N C,H,N+ H,O OH- Answer Bank. chem.libretexts.org › Bookshelves › General_Chemistry14.3: Effect of Concentration on Reaction Rates: The Rate Law Feb 03, 2022 · For each reaction, give the units of the rate constant, give the reaction order with respect to each reactant, give the overall reaction order, and predict what happens to the reaction rate when the concentration of the first species in each chemical equation is doubled. \(\mathrm{2HI(g)}\xrightarrow{\textrm{Pt}}\mathrm{H_2(g)}+\mathrm{I_2(g)} The Chemical Equation - Introductory Chemistry - 1st Canadian Edition Problems. Write and balance the chemical equation for each given chemical reaction. Hydrogen and chlorine react to make HCl. Ethane, C 2 H 6, reacts with oxygen to make carbon dioxide and water.; Solutions. Let us start by simply writing a chemical equation in terms of the formulas of the substances, remembering that both elemental hydrogen and chlorine are diatomic: › read › science8.3 Le Chatelier's principle | Chemical equilibrium | Siyavula The forward reaction is favoured by higher pressures because there are \(\text{2}\) gas molecules of product for every \(\text{4}\) gas molecules of reactant. Refer to Chapter 14 for more information on the Haber process and other industrial applications.
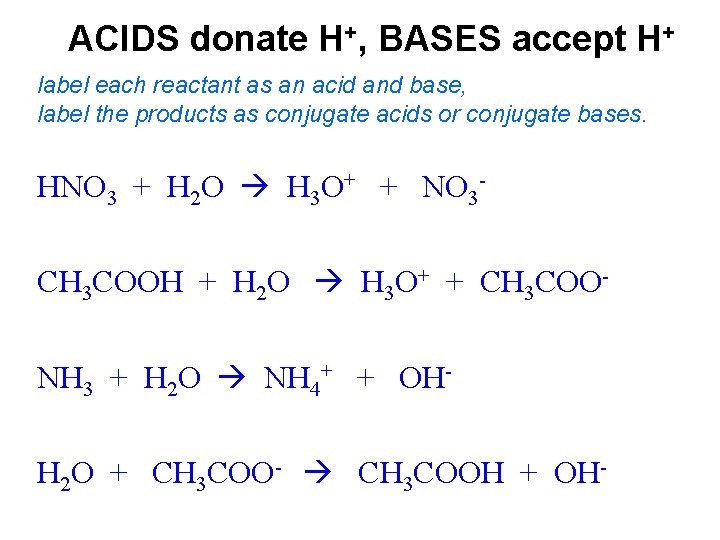
SOLVED:Label each reactant as a Brønsted-Lowry acid or a Brønsted-Lowry ... So in this chemical reaction, we can see that H C N has ah hydrogen ion that it donated. And so it lost it and became C and minus. ... On the product side, the acid produces a conjugal base and the base produces a conjugate acid. In the next chemical reaction, we see that C H three n h two is accepting the hydrogen ion. ... Label each reactant ... Answered: 1 Label each reactant and product in… | bartleby Answered: 1 Label each reactant and product in… | bartleby. Homework help starts here! Science Chemistry Q&A Library 1 Label each reactant and product in this reaction as a Brønsted acid or base. HCN+NH2−↽−−⇀CN−+NH3. 1 Label each reactant and product in this reaction as a Brønsted acid or base. HCN+NH2−↽−−⇀CN−+NH3. Chemical Reactions - Purdue University Chemical reactions are described by chemical equations. Example: The reaction between hydrogen and oxygen to form water is represented by the following equation. 2 H 2 + O 2 2 H 2 O. It is often useful to indicate whether the reactants or products are solids, liquids, or gases by writing an s, l , or g in parentheses after the symbol for the ... Rearrangement - Michigan State University In each case a green capitol letter (C, H, L or S) designates the type of reaction. The first three reactions illustrate the Hofmann rearrangement, which is a particularly useful method for preparing amines. The last example shows a Curtius reaction applied to a diester by way of an intermediate bis-acylhydrazide. An alternative Curtius approach to the amine product of example # 2 is also …
Reactants and Products | Chemistry for Non-Majors | | Course Hero A reactant is a substance that is present at the start of a chemical reaction. The substance (s) to the right of the arrow are called products . A product is a substance that is present at the end of a chemical reaction. In the equation above, the zinc and sulfur are the reactants that chemically combine to form the zinc sulfide product.
Answered: An alkene reacts with water with an… | bartleby Science Chemistry Q&A Library An alkene reacts with water with an acid catalyst results into a formation of: A. Aldehyde B. Ketone C. Alcohol D. Ester 2. 3-Methylhexanal with K2Cr2O7 will yield: A. 3-Methyl-1-hexanol B. 3-Methylhexanoic acid C. 3-Methyl-1-hexanone D. 3-Methyl-1-hexanethiol 3. This is a reverse process of Hydration reaction: A. Oxidation reaction B. …
Chemistry chapter 8 & 9 Flashcards | Quizlet If one knows the mole ratio of a reactant and product in a chemical reaction, one can. calculate the mass of the product produced from a known mass of reactant. Given the equation 3A + 2B --> 2C, the starting mass of A, and its molar mass, and you are asked to determine the moles of C produced, your first step in solving the problem is the ...
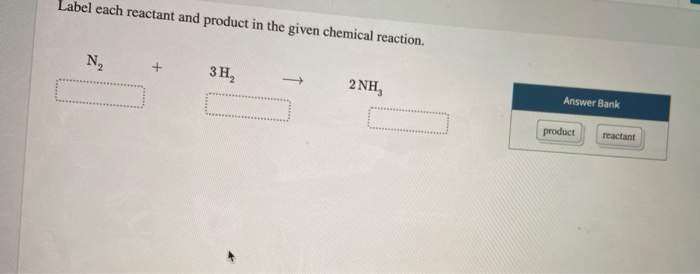
Solved Label each reactant and product in the given chemical | Chegg.com Label each reactant and product in the given chemical reaction. Nt 3H, 2 NH Automobile airbags inflate due to the formation of nitrogen gas from the chemical reaction 2 NaN, (s) + 3N, (B) + 2 Na (s) Identify the number of each atom in the reactants and products for this balanced reaction. Identify the number of Na atoms in the reactants and products.
chem.libretexts.org › Courses › Bellarmine_University5.3: Stoichiometry Calculations - Chemistry LibreTexts The balanced chemical equation was used to calculate the mass of product that is formed from a certain amount of reactant. It can also be used to determine the masses of reactants that are necessary to form a certain amount of product or, as shown in Example \(\PageIndex{1}\), the mass of one reactant that is required to consume a given mass of ...
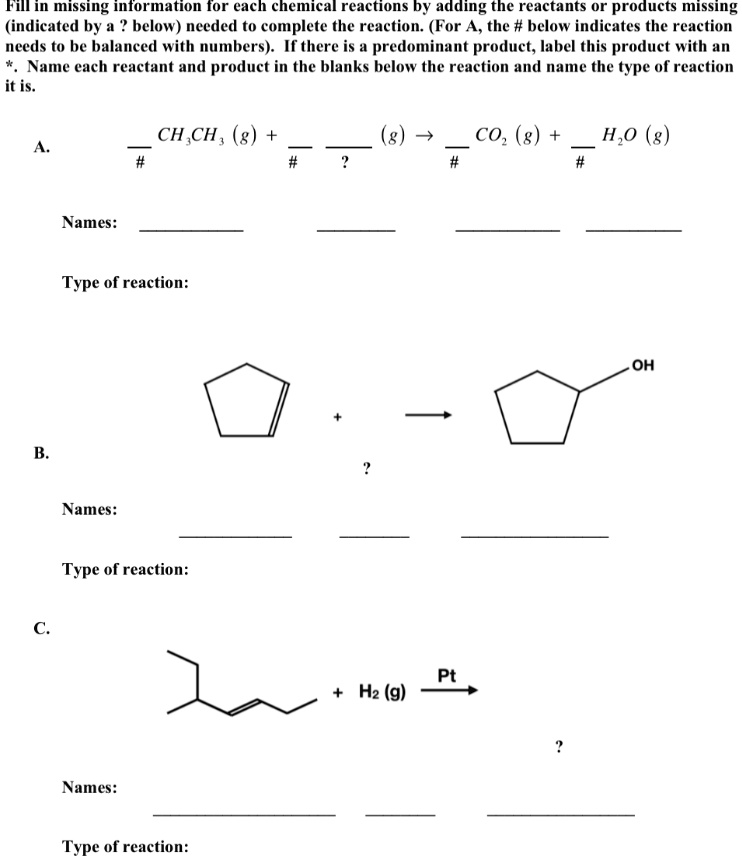
Introduction to Chemical Reactions - GitHub Pages Sulfuric acid is made in a three-step process: (1) the combustion of elemental sulfur to produce sulfur dioxide, (2) the continued reaction of sulfur dioxide with oxygen to produce sulfur trioxide, and (3) the reaction of sulfur trioxide with water to make sulfuric acid (H 2 SO 4 ). Write balanced chemical equations for all three reactions.
en.wikipedia.org › wiki › Kinetic_isotope_effectKinetic isotope effect - Wikipedia The magnitudes of such secondary isotope effects at the α-carbon atom are largely determined by the C α-H(D) vibrations.For an S N 1 reaction, since the carbon atom is converted into an sp 2 hybridized carbenium ion during the transition state for the rate-determining step with an increase in C α-H(D) bond order, an inverse kinetic isotope effect would be expected if only the stretching ...
Chemical Equation | Reactants And Products In Chemical Reactions - BYJUS Law of conservation of mass governs the balancing of a chemical equation. According to this law, mass can neither be created nor be destroyed in a chemical reaction and obeying this law total mass of the elements or molecules present on the reactant side should be equal to the total mass of elements or molecules present on the product side. If the number of atoms of the elements/molecules on the reactant side are not equal to the number of atoms of the elements/molecules on the product side ...
› homework-help › questions-andBackground Kinetics is the study of rates and | Chegg.com It simply represents the overall result of a series of elementary reactions. The reactants may in fact react to form a number of intermediate species which in turn react with each other or more reactant to form the product. The existence of such intermediate species contributes to the overall rate of reaction and may even be ratecontrolling.
Kinetic isotope effect - Wikipedia Background. The kinetic isotope effect is considered to be one of the most essential and sensitive tools for the study of reaction mechanisms, the knowledge of which allows the improvement of the desirable qualities of the corresponding reactions.For example, kinetic isotope effects can be used to reveal whether a nucleophilic substitution reaction follows a …
How would you label each formula in the chemical equation below as ... Explanation: The reactants are on the LEFT HAND SIDE of the equation. The products are on the right hand side. So for the oxidation of iron, F e +S → F eS. Iron and sulfur are the reactants, and iron sulfide is the product.
Reactants and Products - GeeksforGeeks Carbon dioxide and water vapour are the byproducts. When methane gas is burned, the reactants are methane (CH 4) and oxygen in the air (O 2 ). The reaction produces carbon dioxide (CO 2) and water (H 2 O). The reactants in the formation of water are hydrogen (H2) and oxygen (O 2) gas. Water is the product (H 2 O).
Chapter 5: Chemical Reactions Flashcards | Quizlet Study with Quizlet and memorize flashcards terms like WHen the coefficient "4" precedes the reactant NaClO3 in a balanced chemical equation, there are _____ O atoms. - 3 - 4 - 12 - 20, Place the following calculations in the correct order to determine the formula weight of H2SO4. - Formula weight of H2SO4 = 98.09 g/mol - Formula weight of H2SO4 = (2 x M of H) + (M of S) + (4 x M of O ...
Reactants and Products in Chemical Reactions - dummies Methane and oxygen (oxygen is a diatomic — two-atom — element) are the reactants, while carbon dioxide and water are the products. All the reactants and products are gases (indicated by the g's in parentheses). In this reaction, all reactants and products are invisible. The heat being evolved is the clue that tells you a reaction is taking place.
Lab Report Types of Reactions.pdf - Neel Raje Ms. Missig... Ø Record the appearance (colors, texture) of the reactants in the data table. Ø Label each reactant type. Then, choose the type of reaction you expect for the reactants. Ø Use tongs to place a piece of zinc metal into the copper sulfate solution. Allow the two substances to react for a few minutes.
en.wikipedia.org › wiki › Half-lifeHalf-life - Wikipedia For a first-order reaction, the half-life of a reactant is independent of its initial concentration. Therefore, if the concentration of A at some arbitrary stage of the reaction is [A], then it will have fallen to 1 / 2 [A] after a further interval of (ln 2)/k. Hence, the half-life of a first order reaction is given as the following:
› questions-and-answers › completeAnswered: Complete reaction below by typing the… | bartleby A: The reagents (a), (b) and product (c), (d) can be predicted by analysing the product, reagent and… Q: Choose from A-E the term that best describes the isomeric relationship for each of the following…
8.3 Le Chatelier's principle | Chemical equilibrium | Siyavula Le Chatelier's Principle helps to predict what effect a change in temperature, concentration or pressure will have on the position of the equilibrium in a chemical reaction. This is very important, particularly in industrial applications, where …
Answered: 3 > Label each reactant and product in… | bartleby Solution for 3 > Label each reactant and product in this reaction as a Brønsted acid or base H CH,O +H,O CH,ОН + ОН + H,0 3. Answer Bank base acid about us…
Answered: For the reaction scheme below, label… | bartleby Science Chemistry Q&A Library For the reaction scheme below, label each reactant as either a Lewis acid/base (LA or LB) or a Bronsted-Lowry acid/base (BLA or BLB). Use the boxes directly above each reactant for your answer. You only need to write LA or LB or BLA or BLB for your answer. Then, answer the questions below regarding this reaction.
CH104: Chapter 5 - Chemical Reactions - Chemistry This should be the first consideration when writing a chemical reaction.The second consideration should be the states of the matter involved in the chemical reaction. Label each as a solid (s), a liquid (ℓ), a gas (g), or an aqueous solution (aq).
7.2 Reactants and products | Chemical reactions | Siyavula A chemical bond is a force which holds the atoms together. Therefore, during a chemical reaction, the bonds between atoms have to break so that the atoms can rearrange to form the products. New bonds form between the atoms in the product. Next we will look at a chemical reaction that has been used by humankind for centuries.
Controlling the Amount of Products in a Chemical Reaction Engage Have students look at the chemical equation for the vinegar and baking soda reaction as you discuss the reactants. Remind students that in the last lesson, they learned that in a chemical reaction, certain atoms in the reactant molecules unbond from one another and then rearrange and rebond in different ways to form the products.
Worked example: Calculating amounts of reactants and products A balanced chemical equation shows us the numerical relationships between each of the species involved in the chemical change. Using these numerical relationships (called mole ratios), we can convert between amounts of reactants and products for a given chemical reaction.
[Solved] ) In the reaction below, label each reactant as a nucleophile ... Question Answered step-by-step ) In the reaction below, label each reactant as a nucleophile or an electrophile. CH3COO- + O2S (OCH3)2 → CH3COOCH3 + CH3SO4- Chemistry Science Organic chemistry Answer & Explanation Solved by verified expert All tutors are evaluated by Course Hero as an expert in their subject area.
14.3: Effect of Concentration on Reaction Rates: The Rate Law 03/02/2022 · For each reaction, give the units of the rate constant, give the reaction order with respect to each reactant, give the overall reaction order, and predict what happens to the reaction rate when the concentration of the first species in each chemical equation is doubled. \(\mathrm{2HI(g)}\xrightarrow{\textrm{Pt}}\mathrm{H_2(g)}+\mathrm{I_2(g)}
Solved Label each reactant and product in the given chemical | Chegg.com Expert Answer. CH4=REACTANT O2 = REACT …. View the full answer. Transcribed image text: Label each reactant and product in the given chemical reaction. CH_4 + 2O_2 rightarrow CO_2 + 2H_2O.
Answered: Label each reactant and product in this… | bartleby Q: Identify each reactant and product in this reaction as a Brønsted acid or base. H2Y− + H2Z−↽−−⇀H3Y +…. A: Q: From the following list, please choose the conjugate acid of NH3' - CH2 - O NH3* - CHR - COOH O NH3*…. A: Acids are substances that release H+ ions. Bases are substances that accepts H+ ions. Note: Since….





















Post a Comment for "43 label each reactant and product in the given chemical reaction"