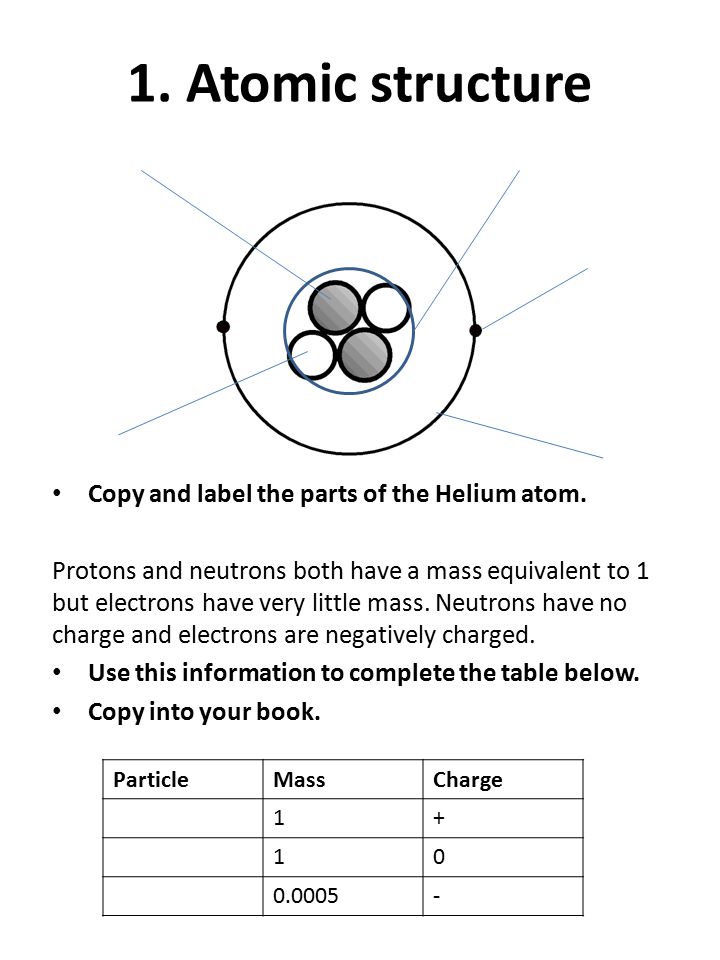
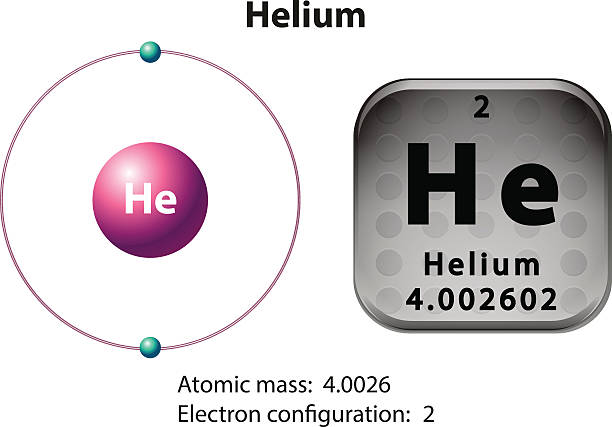
39 helium atom diagram labeled
Helium atom - Wikipedia A helium atom is an atom of the chemical element helium.Helium is composed of two electrons bound by the electromagnetic force to a nucleus containing two protons along with either one or two neutrons, depending on the isotope, held together by the strong force.Unlike for hydrogen, a closed-form solution to the Schrödinger equation for the helium atom has not been found. Atomic Numbers and Electron Configurations Flashcards | Quizlet A. Orbital Size. The electron configuration for Helium (He) is shown below.1s2 Which diagram shows the correct distribution of electrons in the electron shells of a helium atom? C. 2P. 2N. Two representations of the distribution of electrons in the potassium atom are shown. Model 1 Model 2 1s22s22p63s23p64s1.
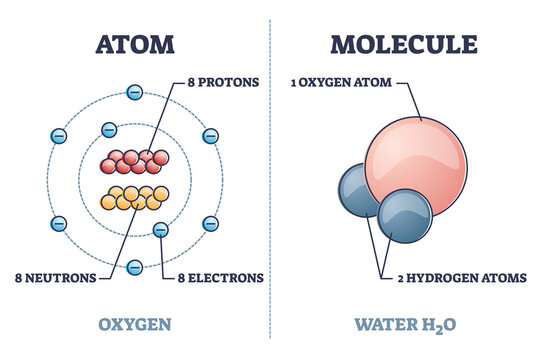
The Structure of an Atom Explained With a Labeled Diagram Basic Diagram of an Atom. Most of an atom is just empty space and consists of a positively charged nucleus of protons and neutrons surrounded by a cloud of negatively charged electrons. The center of an atom is the nucleus and one or more electrons surrounding the nucleus. When one says an atom is electrically neutral, it means that the number ...
Helium atom diagram labeled
8: The Helium Atom - Chemistry LibreTexts The helium atom has two electrons bound to a nucleus with charge Z = 2. The successive removal of the two electrons can be diagrammed as. The first ionization energy I1, the minimum energy required to remove the first electron from helium, is experimentally 24.59 eV. The second ionization energy, I2, is 54.42 eV. Atom Diagrams: Electron Configurations of the Elements - ThoughtCo For each electron shell atom diagram, the element symbol is listed in the nucleus. The electron shells are shown, moving outward from the nucleus. The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. The element atomic number and name are listed in the upper left. Helium - Periodic Table Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure.The chemical symbol for Hydrogen is H. With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table. Its monatomic form (H) is the most abundant chemical substance in the Universe, constituting roughly 75% of all baryonic mass.
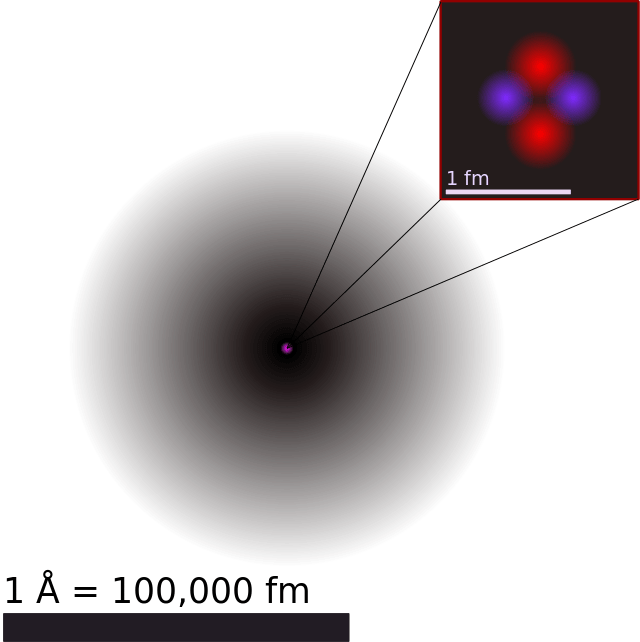
Helium atom diagram labeled. 2.2: Electron Configurations - Chemistry LibreTexts By Hund's rule, the electron configuration of carbon, which is 1 s2 2 s2 2 p2, is understood to correspond to the orbital diagram shown in c. Experimentally, it is found that the ground state of a neutral carbon atom does indeed contain two unpaired electrons. Exercise 2.2.1. Draw an orbital diagram for nitrogen, Z = 7. Atomic radius - Wikipedia Atomic radius. Diagram of a helium atom, showing the electron probability density as shades of gray. The atomic radius of a chemical element is a measure of the size of its atom, usually the mean or typical distance from the center of the nucleus to the outermost isolated electron. Since the boundary is not a well-defined physical entity, there ... How to Write the Atomic Orbital Diagram for Helium (He) To write the orbital diagram for the Helium (He) first we need to write the electron configuration for just He. To do that we need to find the number of ele... Bohr Diagrams of Atoms and Ions - Chemistry LibreTexts Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Figure 2 contrast the Bohr diagrams for lithium, fluorine and aluminum atoms. The shell closest to the nucleus is ...
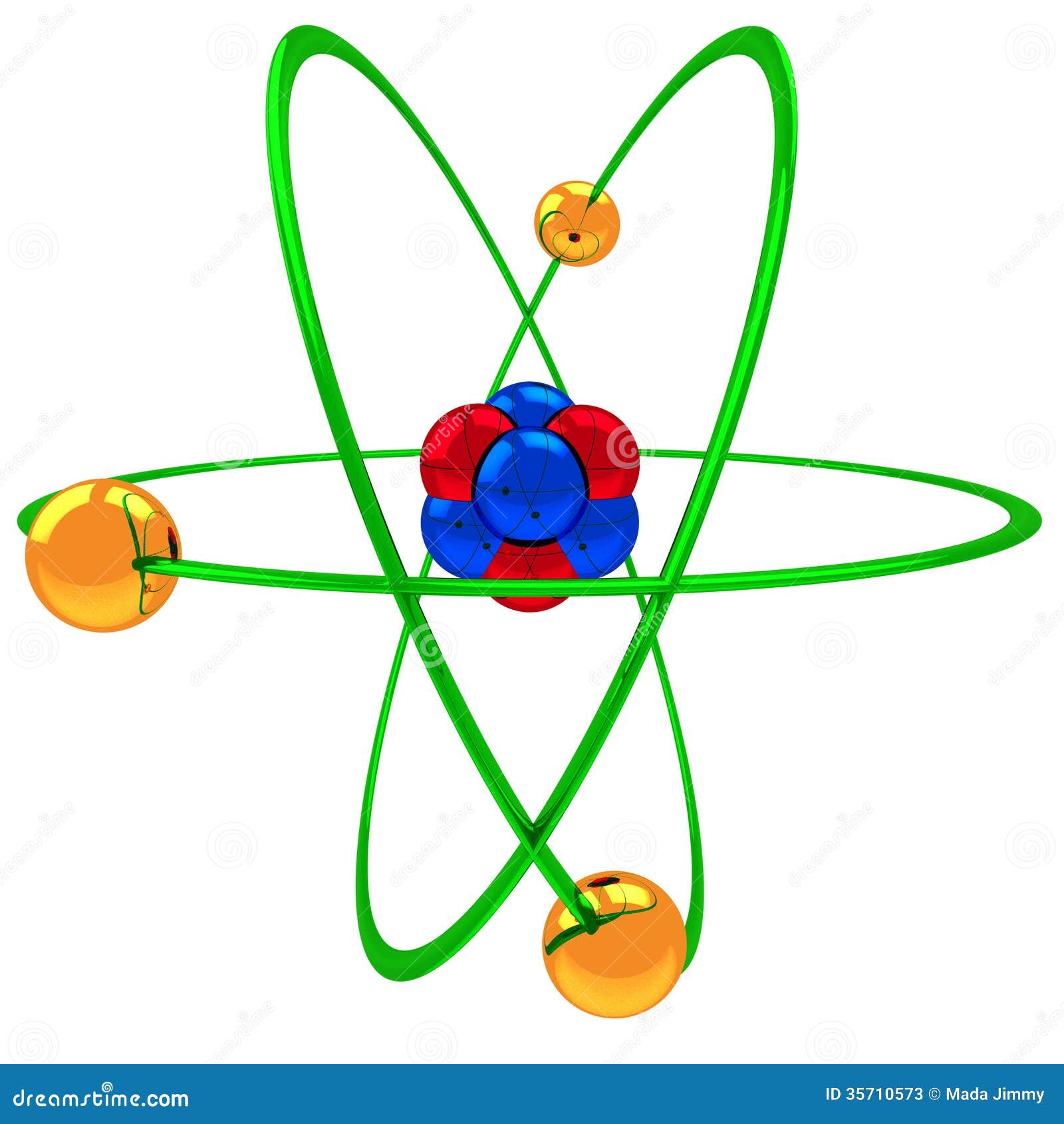
8.4: Molecular Orbital Theory - Chemistry LibreTexts A helium atom has two electrons, both of which are in its 1s orbital. ... The second diagram is similar to the first, but the band gap is about half as large. This diagram is labeled, "Semiconductor." The third diagram is similar to the other two, but the band gap is about a fifth that of the "Semiconductor" diagram. This diagram is ... How to draw Bohr diagram for Helium(He) atom - Topblogtenz The Bohr model of Helium is drawn with only one electron shell and it contains 2 electrons. Helium is neutral and its atomic number is 2, hence, the number of protons and electrons available for its Bohr diagram is also 2. The number of neutrons for the Bohr diagram of Helium can be found by subtracting the number of protons from the atomic ... 9.3: Drawing Lewis Structures - Chemistry LibreTexts Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.". PDF 24. The Helium Atom - Weber State University ground state and the two lowest excited states of helium. To put these results into context, please look at the energy level diagram in Section 5.2.1 of Gri ths. This truncated-matrix approach to the helium atom, including the Mathematica code that I'll show in class, is based on a recent article by Robert C. Mass e and
Helium Atom - an overview | ScienceDirect Topics Find the average mass of a helium atom from its atomic mass (A = 4.003 g mol −1 ÷ 6.022×10 23 atoms mol −1 ÷ 1000 g kg −1 = 6.647×10 −27 kg). Combine equations (2-1) and (2-3) to get. 3. The temperature of a perfect vacuum would be absolute zero because where there is no matter, so there can be no kinetic energy. Helium energy level diagram (from Tanner et al. 2000) The helium states and energy levels can be classified as follows: (i) the ground state and bound singly excited states, (ii) doubly excited resonant states, and (iii) unbound continuum states at ... Helium | He - PubChem Helium | He | CID 23987 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more. ... atom contains an even number of fermions, it is a boson. ... The helium-3 atom contains an odd number of fermions; thus it is ... 1.8: Helium Atom - Chemistry LibreTexts Spinorbitals and the Exclusion Principle. The simpler wavefunctions for helium atom in Equation \(\ref{5}\), can be interpreted as representing two electrons in hydrogen-like 1s orbitals, designated as a 1s 2 configuration. According to Pauli's exclusion principle, which states that no two electrons in an atom can have the same set of four quantum numbers, the two 1s electrons must have ...

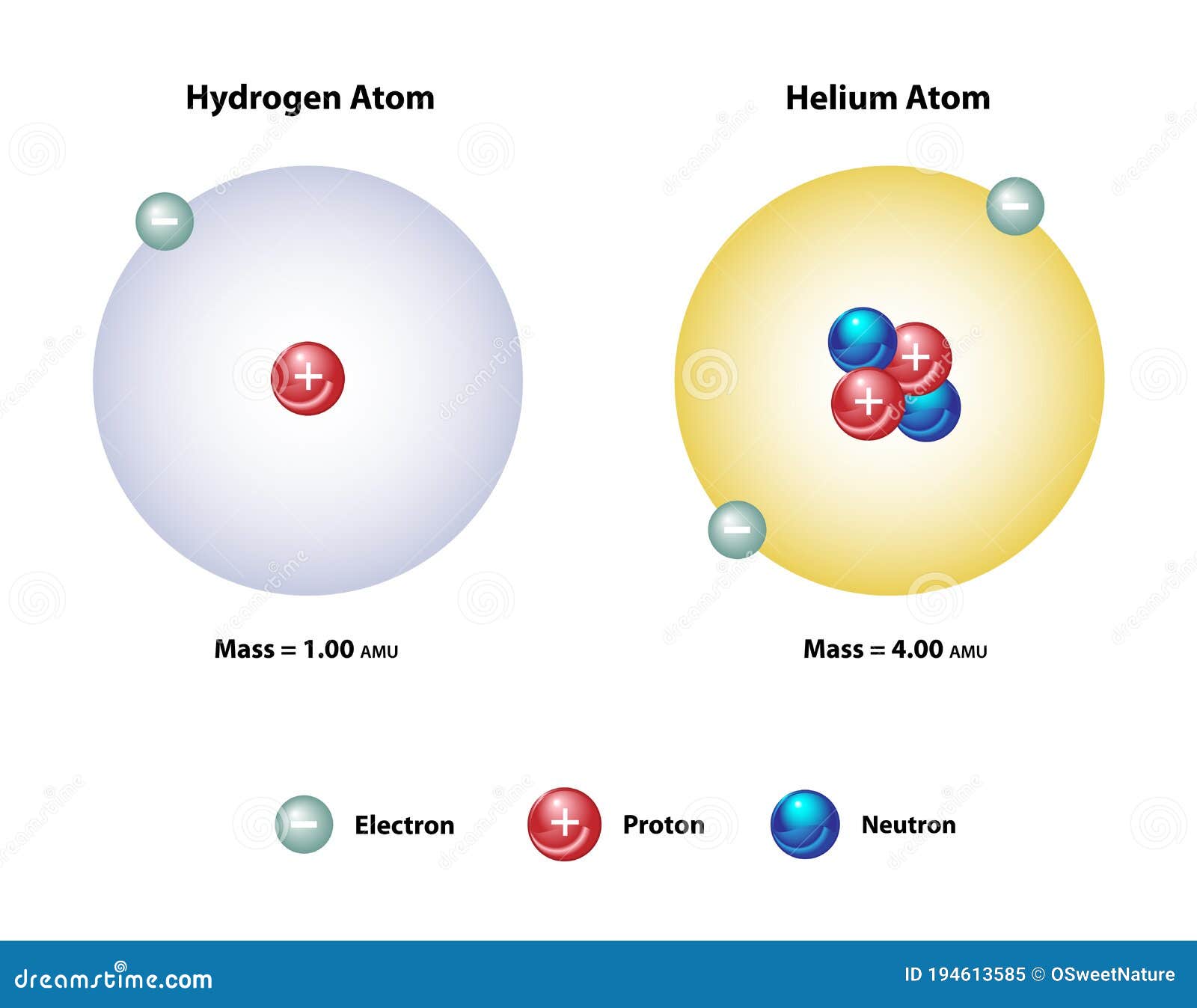
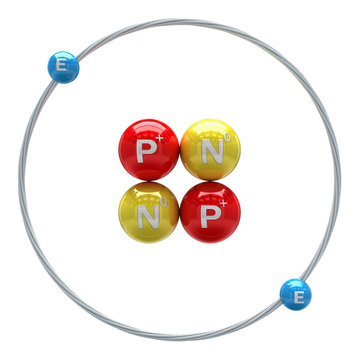
Each helium atom has two protons. Sketch models of helium-3 and helium-4 which have approximate masses of 3 amu and 4 amu, respectively. Label and differentiate protons, neutrons, and electrons in ...
Helium | Definition, Properties, Uses, & Facts | Britannica helium (He), chemical element, inert gas of Group 18 (noble gases) of the periodic table. The second lightest element (only hydrogen is lighter), helium is a colourless, odourless, and tasteless gas that becomes liquid at −268.9 °C (−452 °F). The boiling and freezing points of helium are lower than those of any other known substance. Helium is the only element that cannot be solidified ...
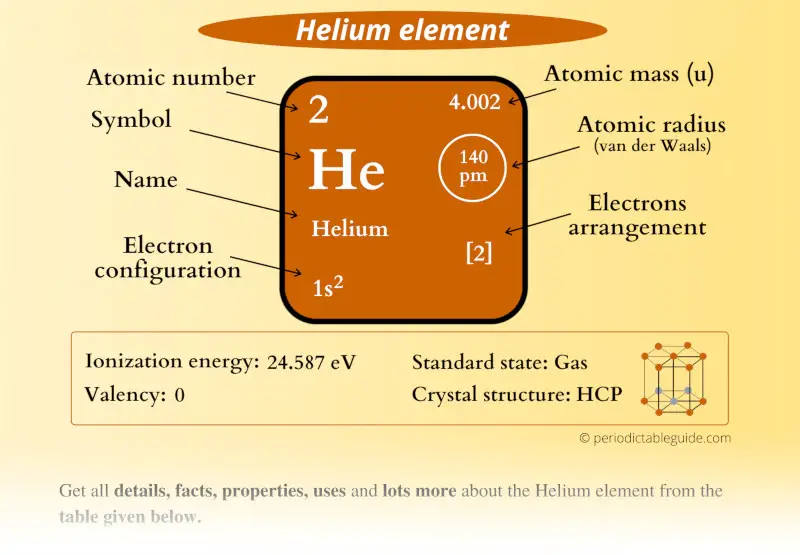
Helium(He) electron configuration and orbital diagram The maximum electrons holding capacity in N orbit is 2n 2 = 2 × 4 2 = 32. Therefore, the maximum electron holding capacity in the first shell is two, the second shell is eight and the 3rd shell can have a maximum of eighteen electrons. Helium electron configuration (Bohr model) The atomic number is the number of electrons in that element.
Helium - Periodic Table Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure.The chemical symbol for Hydrogen is H. With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table. Its monatomic form (H) is the most abundant chemical substance in the Universe, constituting roughly 75% of all baryonic mass.
Atom Diagrams: Electron Configurations of the Elements - ThoughtCo For each electron shell atom diagram, the element symbol is listed in the nucleus. The electron shells are shown, moving outward from the nucleus. The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. The element atomic number and name are listed in the upper left.
8: The Helium Atom - Chemistry LibreTexts The helium atom has two electrons bound to a nucleus with charge Z = 2. The successive removal of the two electrons can be diagrammed as. The first ionization energy I1, the minimum energy required to remove the first electron from helium, is experimentally 24.59 eV. The second ionization energy, I2, is 54.42 eV.




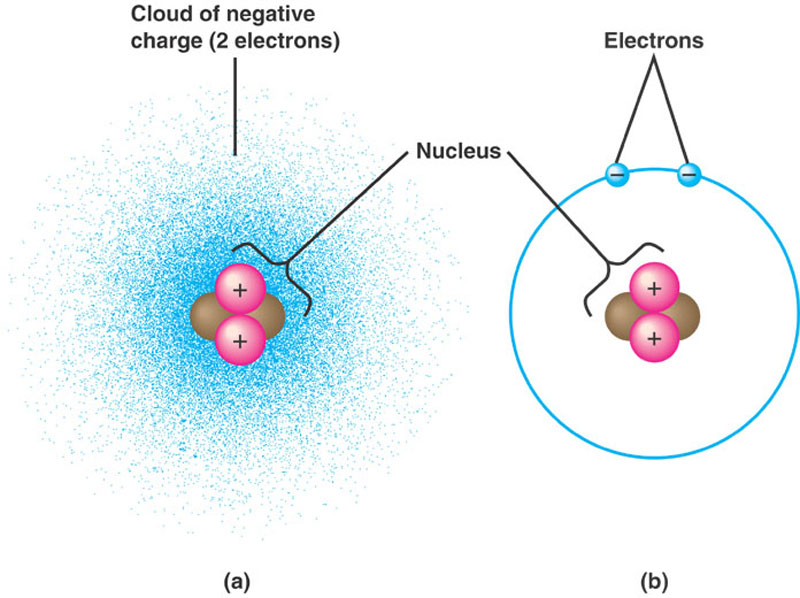
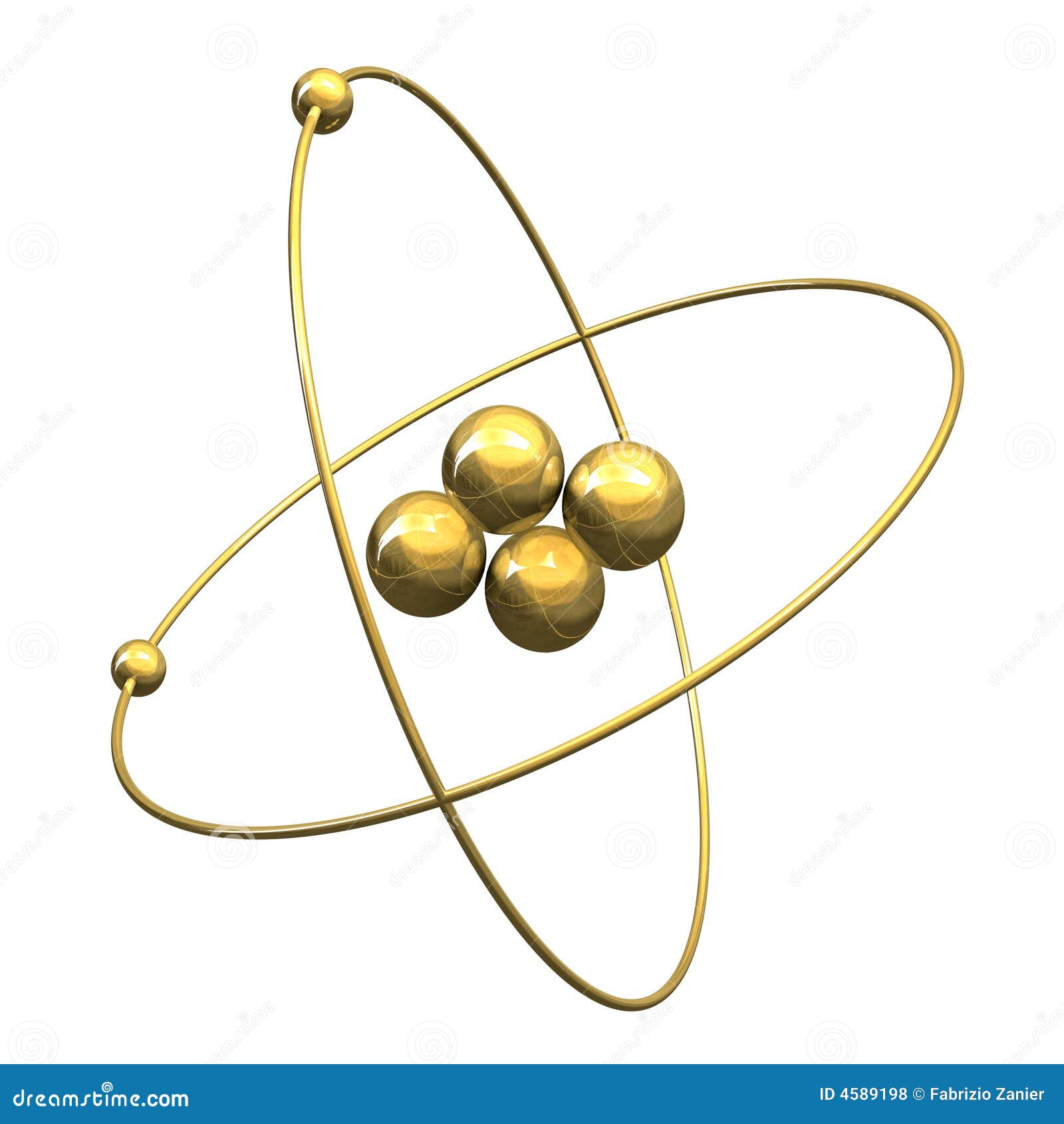





















Post a Comment for "39 helium atom diagram labeled"