41 open-label study advantages and disadvantages
(PDF) What is an open label trial? - ResearchGate The small sample size and the dropout rate of 40% limit the generalisability of the results, as well as increase the risk of type 2 errors (Sullivan & Feinn, 2012). Another limitation is linked to... › about-cancer › treatmentFatigue (PDQ®)–Health Professional Version - NCI Sep 01, 2022 · On the basis of two promising open-label pilot trials,[27,28] a large randomized controlled trial evaluated modafinil for the treatment of CRF using 200 mg versus placebo in more than 850 patients who were receiving chemotherapy. Patients had to have fatigue ratings of at least 2 out of 10 to be eligible for this study.
Open-label extension studies: do they provide meaningful ... - PubMed Negative aspects of open-label extension studies revolve around their use as a marketing tool, as they build a market for the drug and generate pressure for subsidised access to the drug from consumers and their physicians.
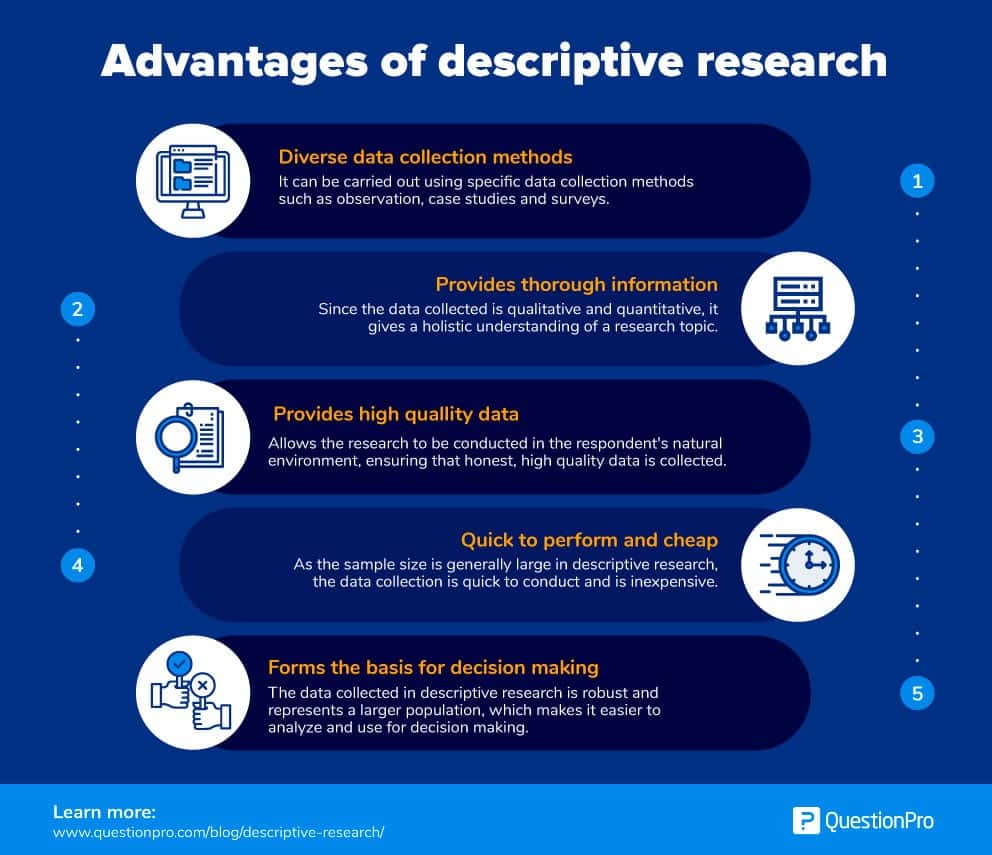
Open-label study advantages and disadvantages
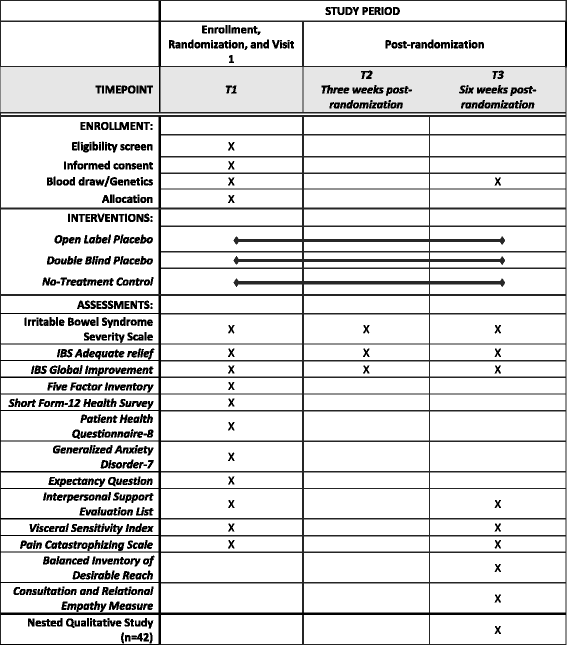
Effects of Open- and Closed-Label Nocebo and Placebo Suggestions on ... These so-called open-label placebo effects have been found to significantly reduce symptoms of irritable bowel syndrome, depression, attention deficit hyperactivity disorder, chronic low back pain, and allergic rhinitis ( 31 - 39 ). reliefweb.int › job › 3890555RCT specialist for the Evaluation of the MSF TB-Practecal ... Sep 28, 2022 · Linked to the previous question, the evaluation will explore alternatives to the chosen design, if any, and elaborate on potential advantages and disadvantages. Open-Label Extension Studies | SpringerLink The reputation of open-label extension study has been damaged by studies for which this has been the motivation. They have commonly been distinguished by rudimentary data collection and quality, revealing the true motive, namely market expansion.
Open-label study advantages and disadvantages. PDF Labeling and Disadvantages of Labeling - University of North Carolina ... scientists would be in raising money for cancer research if they had no name for it. The advantages of labeling can be summarized as follows: 1. Federal and local funding of special education programs are based on categories of disabilities. 2. Labeling enables professionals to communicate with one another because each categorical label en.wikipedia.org › wiki › Meta-analysisMeta-analysis - Wikipedia The inverse of the estimates' variance is commonly used as study weight, so that larger studies tend to contribute more than smaller studies to the weighted average. Consequently, when studies within a meta-analysis are dominated by a very large study, the findings from smaller studies are practically ignored. A Comparison of Advantages and Disadvantages of Opening a Sole ... When people decide that they want to open up a restaurant or something similar, there are a lot of important decisions that they need to make. One of the most important decisions that need to make is whether they want to open up a franchise restaurant or a sole proprietorship and make their own restaurant. There are a lot of contributing factors that determine which would be the best option ... Cardiovascular clinical trials in Japan and controversies ... - Nature Table 1 Advantages and disadvantages of the PROBE design compared with double-blinded design. ... (Jikei Heart Study): a randomised, open-label, blinded endpoint morbidity-mortality study.
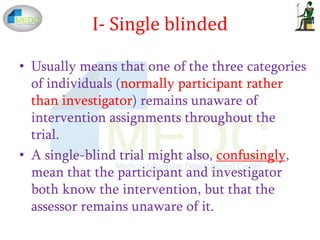
External and internal validity of open label or double‐blind trials in ... Naturally, in open-label trials in anticoagulation there is a risk of a reporting bias of adverse events. Patients may research the new drug and its side-effects in publications and may be influenced in their reporting behaviour of potential side-effects. Furthermore, investigators may be equally susceptible to a reporting bias. Study in China: advantages and disadvantages (Education in China) Education in China: advantages and disadvantages. When a person plans to study abroad, popular European universities immediately come to mind. At the same time, Chinese universities are in the TOP 3 countries, whose graduates find it easier to get a well-paid job. But studying in China also has other benefits: Open-label study | definition of open-label study by Medical dictionary open-label study a study in which there is no blinding of treatments. Farlex Partner Medical Dictionary © Farlex 2012 open-label study A clinical study in which the patients/subjects and investigators know which product each patient/subject is receiving, which is the opposite of a blinded study. Segen's Medical Dictionary. © 2012 Farlex, Inc. Reducing bias in open-label trials where blinded outcome assessment is ... This can be an issue in trials assessing surgical interventions, device trials, or other non-pharmacologic interventions, which are more difficult to blind than traditional drug trials [ 4 ]. Many such trials are therefore open-label, where patients, clinicians, and care providers are aware of treatment allocations.
External and internal validity of open label or double-blind ... - PubMed In general, a blinded trial is regarded as being less subject to bias than an open trial because it minimizes the impact of knowledge of treatment allocation on post-randomized treatment decisions and on reporting of outcomes. However, a blinded trial is not always feasible. en.wikipedia.org › wiki › Analysis_of_clinical_trialsAnalysis of clinical trials - Wikipedia If a patient drops out of the study after the third week, then this value is "carried forward" and assumed to be his or her score for the 5 missing data points. The assumption is that the patients improve gradually from the start of the study until the end, so that carrying forward an intermediate value is a conservative estimate of how well ... External and internal validity of open label or double ... - ResearchGate a blinded trial may be less subject to bias than an open-label trial because it minimizes the impact of knowledge of treatment allocation on postrandomized treatment decisions and on reporting of... National Cancer Institute NCI's Dictionary of Cancer Terms provides easy-to-understand definitions for words and phrases related to cancer and medicine.
Open-Label Trial - an overview | ScienceDirect Topics Open-label studies lack the rigor of blinded studies. Since the lack of blinding can introduce significant bias, reserve the use of open-label studies for situations in which blinding is neither feasible nor ethical or in cases where the outcome is completely objective, such as survival. Some situations include: •
Franchising: Advantages and Disadvantages | Free Essay Example Advantages of franchising. One of the merits of franchising is that most franchisees record impressive profits especially when managed effectively. It is also prudent to mention that both the capital and earnings can be boosted at the same time due to the available opportunities. Decision making is expedited because the owner can make direct ...
What is an Open-Label Clinical Trial? - News-Medical.net Open-label trials can be used to gather additional safety and efficacious data on drugs on the market to increase the confidence of clinicians, patients, and clinical bodies. They can play a key ...
Advantages And Disadvantages Of Private Own Labels | Bartleby The advantages of own label it is included: 1- It helps to focusing on the need of the target consumer, and uses the intelligence of the store to understanding the manners and preferences of the consumer. 2- It enables the direct relationship with manufacturers and suppliers, to reduce the distance, and improve traceability.
External and internal validity of open label or double-blind trials in ... disadvantages of open-label or double-blind trials is currently underway and interpretation oftrialresults isoften focusedon this matter. In general, a blinded trial is regarded as being less subject to bias than an open trial because it minimizes the impact of knowledge of treatment allocation on post-random-
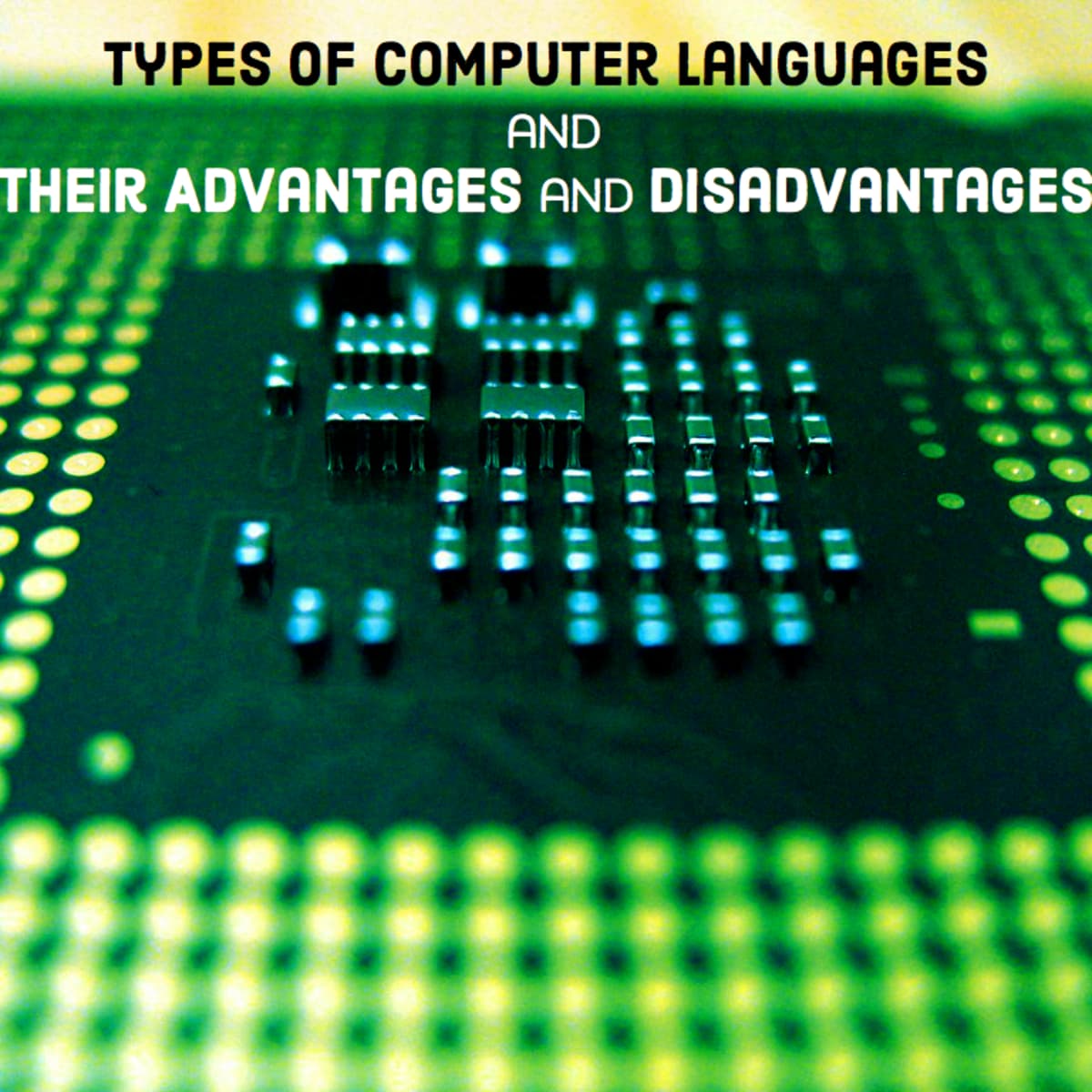
› pmc › articlesDirect Oral Anticoagulants: A Quick Guide - PMC Nov 29, 2016 · In the XANTUS study, patients had a mean CHADS 2 score of 2 compared to 3.5 in the ROCKET-AF study, which could explain the discrepancy in the rates of thrombotic complications. Apixaban was shown to be non-inferior (p<0.001) and superior to warfarin (p<0.01) for the prevention stroke and systemic embolism in the Apixaban for Reduction in ...
Study designs — Centre for Evidence-Based ... - University of Oxford An experimental comparison study in which participants are allocated to treatment/intervention or control/placebo groups using a random mechanism (see randomisation). Best for study the effect of an intervention. Advantages: unbiased distribution of confounders; blinding more likely; randomisation facilitates statistical analysis.
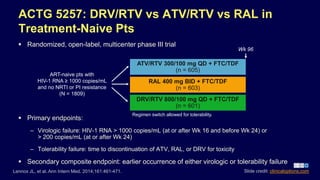
14 Advantages and Disadvantages of a Randomized Controlled Trial - Vittana List of the Disadvantages of Randomized Controlled Trials 1. The logistics of a randomized controlled trial can be demanding. Researchers and participants may need to endure a long trial run to ensure that there is enough data for comparison.
Open-Label Trial - an overview | ScienceDirect Topics An open-label trial has no blinding: everyone knows which patient is receiving which treatment. Open-label studies lack the rigor of blinded studies.
Effectiveness studies: advantages and disadvantages - PMC - Cannot make as meaningful clinical comparisons between agents Highly restricted inclusion criteria to reduce confounding biases Randomization and blinding, also to reduce bias - Fewer treatment adjustments are allowed - Higher internal validity for clinical effects - Higher internal validity for adverse effects, tolerability
Self-Manage Scleroderma | Lesson There are some advantages and disadvantages to being in a clinical trial The advantages include: Patients will receive the best standard treatment. If the new treatment or intervention is shown to work, patients may be among the first to benefit. Patients have a chance to help others and improve patient care. Some disadvantages might be:
What is an open label trial? | The BMJ Open label trials are sometimes referred to as "non-masked" or "unblinded.". If the trial is a non-pharmacological study, such as a trial of devices, or psychological and physical treatments, it may be referred to simply as "open.". After recruitment to the trial, the participants were allocated to treatment using block randomisation.
› en › public-healthGuidance on use of Imvamune in monkeypox outbreaks in Canada ... Aug 03, 2022 · An Open-Label Phase II Study to Evaluate Immunogenicity and Safety of a Single IMVAMUNE Booster Vaccination Two Years After the Last IMVAMUNE Vaccination in Former POX-MVA-005 Vaccinees [Internet]. Bethesda (MD): ClinicalTrials.gov [updated 2019 Mar 13; cited 2022 Jun 2].
Crossover trials: what are they and what are their advantages and ... One can say that study participants serve as their own control. This leads to another advantage which is less study participants are required compared to a standard parallel randomized controlled trial (RCT). Reduction of sample size is consistent with the principle in medical research to use resources wisely.
› articles › 295714Yogurt: Types, health benefits, and risks - Medical News Today Jan 11, 2018 · Yogurt is packed with nutrients that can include calcium and magnesium, good bacteria, and protein. But not all yogurts are as healthy as each other. In this article, we explain the good and the ...
Open-label trial - Wikipedia Open-label trials may be appropriate for comparing two similar treatments to determine which is most effective, such as a comparison of different prescription anticoagulants, [4] or possible relief from symptoms of some disorders when a placebo is given. [5] An open-label trial may still be randomized.
Open-Label Trial | NIH - HIV.gov A type of clinical trial. In open-label trials, both the researchers and participants know which drug (or other intervention) is being given to participants.
Understanding Clinical Trial Terminology: What is an Open Label ... Alternatively, sometimes, trials are conducted in an open-label fashion, meaning study participants and researchers both know which treatment the patient is receiving. Open-label trials can be used to compare treatments or gather additional information about the long-term effects in the intended patient population.
Open-Label Extension Studies | SpringerLink The reputation of open-label extension study has been damaged by studies for which this has been the motivation. They have commonly been distinguished by rudimentary data collection and quality, revealing the true motive, namely market expansion.
reliefweb.int › job › 3890555RCT specialist for the Evaluation of the MSF TB-Practecal ... Sep 28, 2022 · Linked to the previous question, the evaluation will explore alternatives to the chosen design, if any, and elaborate on potential advantages and disadvantages.
Effects of Open- and Closed-Label Nocebo and Placebo Suggestions on ... These so-called open-label placebo effects have been found to significantly reduce symptoms of irritable bowel syndrome, depression, attention deficit hyperactivity disorder, chronic low back pain, and allergic rhinitis ( 31 - 39 ).

:max_bytes(150000):strip_icc()/bigger-breasts-without-getting-implants-pros-cons-2709949_final-882aef5dadb74c90ba8e4a47f5728808.jpg)
























Post a Comment for "41 open-label study advantages and disadvantages"